Which of These Is a Property of Bases
Bases produce hydrogen ions H when added to water. Which is a property of all bases.

Ncert Solutions For Class 10 Science Chapter 2 Acids Bases And Salts Learn Insta Class10science Ncertsolutionsforcl Acids Bases And Salts Solutions Science
They turn litmus paper blue.

. Bases react with acids. C They turn blue litmus red. Physical Properties of Bases.
Bases have a bitter taste. Bases dissolve many metals. Some say bases feel slippery or soapy.
Bases taste bitter There are very few food materials that are alkaline. Indicator compounds such as litmus can be used to detect basesBases turn red litmus paper blue. They are bitter to taste and they turn red litmus paper blue but the blue litmus paper remains unaffected.
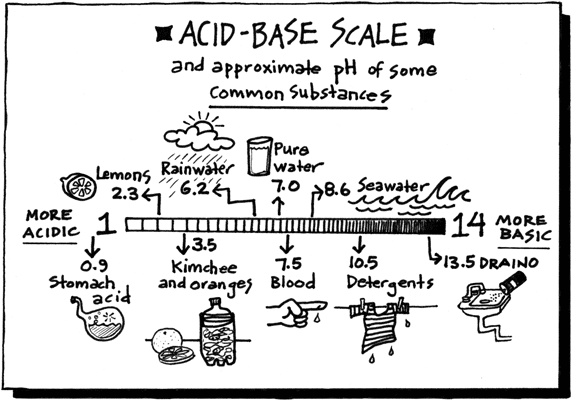
This is because they dissolve the fatty acids and oils on your skin and reduces friction. Start studying Properties of Acids and Bases Assignment and Bases. The strength of bases is measured on the pH scale.
It turs the paper blue is correct. The taste of bases is bitter not sour. Strong bases have three main properties.
Bases form OH ions. B Acids dissolve many metals. Bases release a hydroxide ion in water solution.
Which of the following is NOT a property of bases. Some of the general properties of bases are as follows-. C They turn blue litmus red.
Bases have a bitter taste. They also react with acids to form salts. 2 on a question Which of these is a property of bases.
The only property of bases according to the options given is that they turn litmus paper blue. Which of these is a property of bases. Bases form OH ions.
They produce hydrogen gas when reacted with metals. Learn vocabulary terms and more with flashcards games and other study tools. C Acids have a slippery feel.
Which of these are properties of bases. Which of these is a property of bases. Bases are used in the manufacturing of soap.
Which of these is a unique property of bases. What are the three properties of bases. Bases make indicators change colors.
Turn litmus blue III. 1 and 3 only c. Which of these substances is a base.
Bases are substances that are slippery when touched and change the color of any indicator depending on the color. Describe at least three ways about how allosteric regulation of enzymes works. Correct option is D A.
Acids usually have H as their first element in the formula. DAcids have a sour taste. Which of the following is NOT a property of bases.
They feel soapy to touch. Bases have a slippery feel. Bases do not change the colour of red litmus.
They taste bitter feel slippery and turn litmus paper blue. Which of the following is NOT a property of acids. Bases have a high pH above 7.
They turn red litmus blue. Acids produce H30 in water. Which of these is a property of bases.
Acids cause red litmus indicator dye to turn blue. 1 and 2 only b. Which of the following is a property of a base.
The strength of bases is measured on the pH scale. Tasting of bases is more dangerous than tasting acids because of the property of stronger bases to denturate protein. Fertilizer manufacture and cotton processing use bases.
Indicator compounds such as litmus can be used to detect bases. Bases have a bitter taste and have a slip-pery feel. Myrzilka 38 1 year ago.
Nataly_w 17 1 year ago. Which of the following is a property of a base. All of the above.
A They react with acids and neutralise them. Which of these acids is a component in vinegar. Option A is correct.
A Acids turn litmus paper red. Bases have a sour taste. Bases turn red litmus paper blue.
They turn litmus paper blue. What are the 5 properties of bases. Hydrogen gas when reacted with minerals.
D They have bitter taste. Bases turn red litmus paper blue. It is even more important that care be taken in tasting bases.
B They turn red litmus blue. They produce hydrogen gas when reacted with metals Send. Taste bitter make H in water react with metals react with carbonates Question 38 Which of the following is not a typical property of an acid.
I and III e. EAll of the above are properties of acids. Some of the characteristics of bases include.
Bases have a slippery feel. Which of these is a typical property of bases. Which of the following statements about a base are TRUE.
Bases taste bitter feel slippery and conduct electricity when dissolved in water. Dissolve some metals IV. They release hydroxide ion in their solution medium and they release heat on dilution.
Bases taste bitter feel slippery and conduct electricity when dissolved in water. Which of the following is NOT a property of bases. Weak bases have other properties.
Soldi70 247K 1 year ago. They produce hydrogen gas when reacted with metals. I and II d.
Correct answer to the question Which of these is a property of bases. They turn litmus paper blue. Carboxylic acids can be found in.
You might be interested in. C Acids have a slippery feel.

Acids And Bases Boundless Chemistry

This Will Guide Students Through Reading Information About Acids Bases And The Strength Of Acids And Expository Text Guided Reading Comprehension Worksheets

Properties Of Acids And Bases Physical And Chemical Properties With Examples
Acids And Bases Science With Mrs Beggs

Ncert Solutions For Class 10 Science Chapter 2 Acids Bases And Salts In 2021 Acids Bases And Salts Solutions Science

Chemical Properties Of Acids And Bases Properties Videos And Examples

Properties Of Matter Chemistry Basics Physical And Chemical Properties Chemistry Classroom

The Acid Base Properties Of Water Video Lesson Transcript Study Com

Properties Of Acids And Bases Chemtalk

Acids Bases And Salts Learning Activities Distance Learning Chemistry Activities Common Core Reading Common Core State Standards

Pin On Aio Hospitality Solutions

Can We Cannibalize These Chair Bases For A Round Rolling Platform That Is The Royal Dais And Croaker S Well Canning Conference Room Table Conference Room

Intensive Vs Extensive Properties Chemistry Basics Physical And Chemical Properties Chemistry Classroom




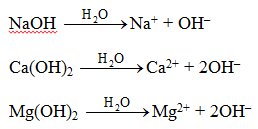
Comments
Post a Comment